(School of Basic Medical Sciences) Recently, Professor Yu Hua from the School of Basic Medical Science of Nanchang University, in collaboration with Professor Zhang Jin's team from Zhejiang University, has established a paper titled "A second-generation M1-polarized CAR macrophage with antitumor efficacy" in the international journal Nature Immunology. In the research, a macrophage-specific CAR (chimeric antigen receptor) and the improved second-generation CAR were designed. Via the differentiation platform, the CAR-expressing iPSCs (induced pluripotent stem cells) were differentiated into the second-generation CAR-iMACs (induced pluripotent stem cell-derived macrophages) with enhanced functions. This work provides insights into the antigen-dependent M1 polarization and activation of the CAR-iMAC and its antitumor mechanism of CAR-mediated efferocytosis of iMACs. The strategy of iPSC differentiation not only promises an abundant and reliable source of therapeutic cells for clinical treatment, but also shows the great potential of CAR-macrophage in clinical applications with its efficacy against solid tumors.

Treating solid tumors effectively has always been one of the significant challenges in medical science. Traditional treatments, such as surgical removal, radiotherapy, and chemotherapy, face limitations due to recurrence, drug resistance, and side effects. While CAR-T (chimeric antigen receptor T cells) therapy has achieved great success in treating hematological malignancies, its effectiveness against solid tumors is less promising. Macrophages play a crucial role in humoral immunity and cellular immunity concerning its functions in immune clearance (defending abnormal cells and pathogens), antigen presentation, and immune regulation. Hence, researches into macrophage against solid tumors hold substantial potential value. However, applying macrophages in the treating still faces numerous challenges.
Against this backdrop, Professor Yu Hua, in collaboration with Professor Zhang Jin's team, has successively developed two generations of CAR-iMAC. The research about the first generation, published in the Journal of Hematology and Oncology in 2020 (IF 28.5), has been cited over 140 times.
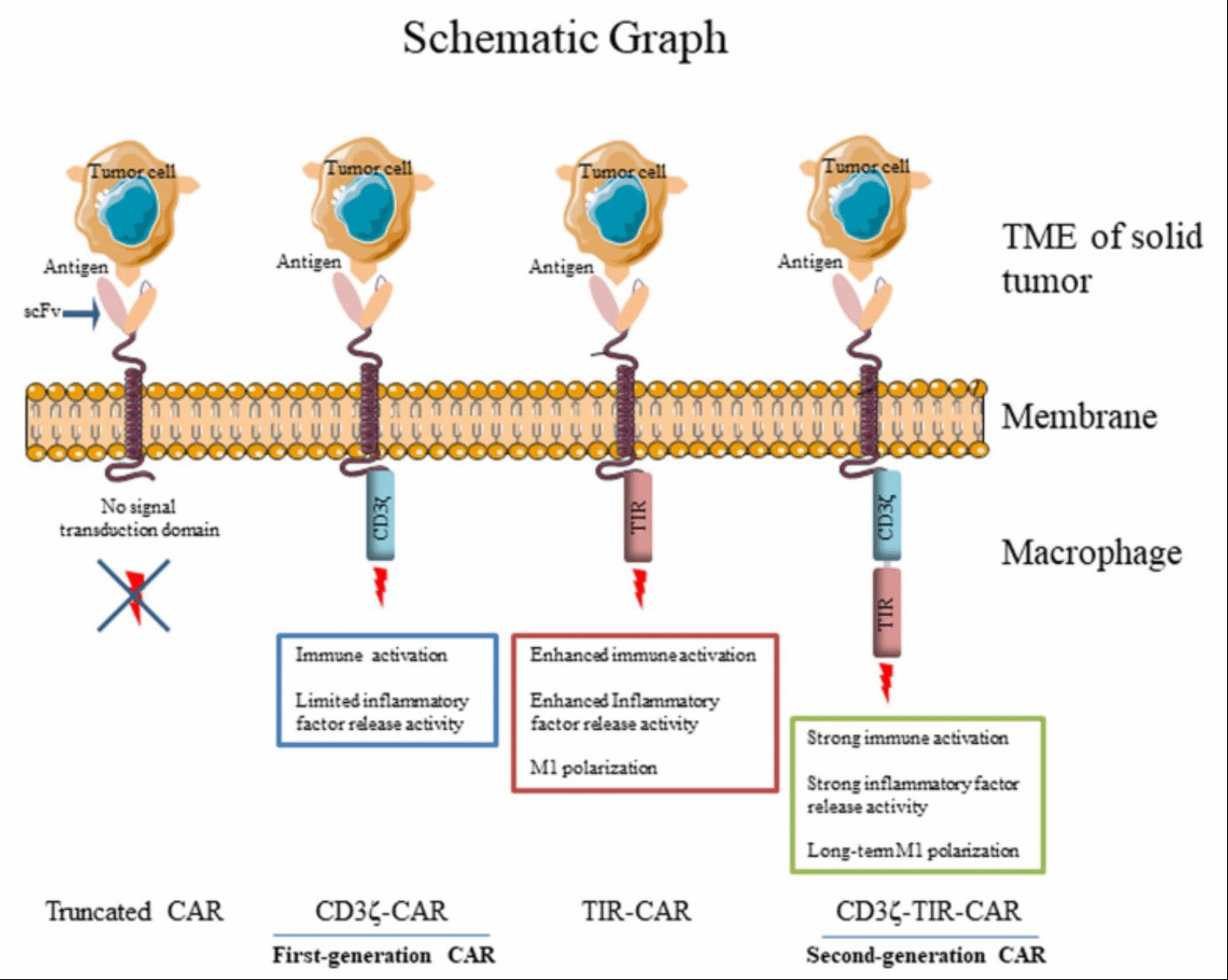
The study of the second-generation CAR-iMAC has recently been published in Nature Immunology. Via the differentiation platform, to which Zhang Jin's team own intellectual property, and by means of genetic engineering, the second-generation with functions of polarization and activation was developed. The findings demonstrated that the second-generation performed better in antigen presentation and resistance to the immunosuppressive tumor microenvironment, maintaining a higher and more prolonged level of M1 polarization in combating tumors. Notably, the treatment with the second-generation CAR-iMAC led to complete remission in hepatocellular carcinoma (HCC)-bearing mice, and significantly prolonged the survival of the glioblastoma (GBM)-bearing mice. This research also sheds light on its mechanism of action against solid tumors, particularly through inducing tumor cell apoptosis and clearing apoptotic bodies via efferocytosis.
In developing both generations of CAR-macrophages, Professor Yu Hua undertook the entire task of data mining. His analysis of the differentiation trajectory and omics characteristics of CAR-macrophages affirmed the rationality and feasibility of the genetically engineered second-generation CAR-iMAC, and laid a foundation for the further modification and upgrade.
About the Author

Yu Hua, a Ph.D. in Bioinformatics, is a distinguished Professor and Doctoral Supervisor at the School of Basic Medical Sciences of Nanchang University. Professor Yu has long been committed to elucidate the functions and mechanisms of non-coding RNA in totipotent stem cells and human cancers by combining both dry and wet research strategies, and discover key non-coding RNAs driving the fate transition of totipotent stem cells and the occurrence and development of human cancers, so as to further screen potential drugs targeting key non-coding RNAs. In recent years, he has led 5 provincial and ministerial-level scientific research projects, published over 20 SCI-indexed papers in prestigious journals such as Nature Communications, Nature Immunology, Nature Metabolism, Protein & Cell, Genome Biology, and Bioinformatics, and developed 7 bioinformatics software. He has been invited to give a presentation about his representative research findings at the International Society for Stem Cell Research (ISSCR) annual meeting. Two of his papers were selected as journal covers, and one paper was featured in Science News and other renowned media outlets.
Editor: Cheng Huiping
Executive Editor: Tu Jinfeng




