On August 13th, Professor Zhang Jin from the School of Basic Medical Sciences of our school cooperated with Boston Children’s Hospital and Medical School of Harvard University to publish an online research paper entitled “Structure of the mammalian TRPM7, a magnesium channel required during embryonic development”on PNAS.
This is the third important scientific achievements of the research team following the publication on PNAS in February and Nature Communications last week. Professor Zhang Jin and Professor David Clapham are co-corresponding authors of the paper.
Stroke is the second most deadly disease that threatens human health as millions of people died every year because of it. This devastating disease often occurs without warning, causing permanent disability to the patient and depriving their ability to live an independent life, and placing a heavy burden on the patient’s family and society. There are nearly 70 million stroke patients in China, 80% of which are caused by ischemic stroke. Therefore, drug research and development for ischemic stroke has been the focus and hotspot of the research. However, there are currently no drugs that are very effective against ischemic stroke. The glutamate NMDA receptor is the most common target drugs for stroke because its regulated excitotoxicity causes ischemic neuronal death, and antagonists can block this process and prevent ischemic stroke. However, the wide distribution of NMDA receptors in the body and the diversity of their neurological functions, their targeted drugs have different degrees of side effects for stroke patients. Therefore, people have been seeking more effective and specific drug targets.
TRPM7 is a non-selective cation channel. When the concentration of extracellular calcium or magnesium ions decreases, the TRPM7 ion channel is activated and turned on, causing calcium overload during ischemia, which causes nerve cell death. Inhibition of TRPM7 in vivo blocks this process, which greatly reduces neuronal cell death caused by ischemia and protects neurons over a longer period of time. At the same time, the activation and inhibition signaling pathway of TRPM7 is not affected by the NMDA receptor signaling pathway. it can be predicted that the corresponding antagonists of TRPM7 target’s protective effect on neurons is more effective and durable than existing drugs. In summary, TRPM7 is an ideal target for the treatment of ischemic stroke. The development of selective inhibitors TRPM7 target will provide new ideas for drug development for stroke, and can analyze the structure of TRPM7 based on its structural development. Related small molecule inhibitors are of great importance for the development of drugs for stroke treatment.
Magnesium ions play an important role in the life activities of the human body and are the main divalent cations in the cells. The complex of magnesium ions and ATP is an important co-factor for enzymes and nucleic acids required for cell function, replication and energy metabolism. Low levels of magnesium ions induce many chronic and inflammatory diseases such as cardiovascular disease (e.g. stroke), Alzheimer’s disease, diabetes, migraine and osteoporosis. However, little is known about the molecular mechanisms by which magnesium ions are transported in the body. Recent studies have shown that transient receptor potential ion channels TRPM6 and TRPM7 are closely related to the transport of magnesium ions. At the same time, TRPM7 plays a key role in the pathogenesis of cardiovascular diseases by regulating the homeostasis of magnesium ions in vivo.
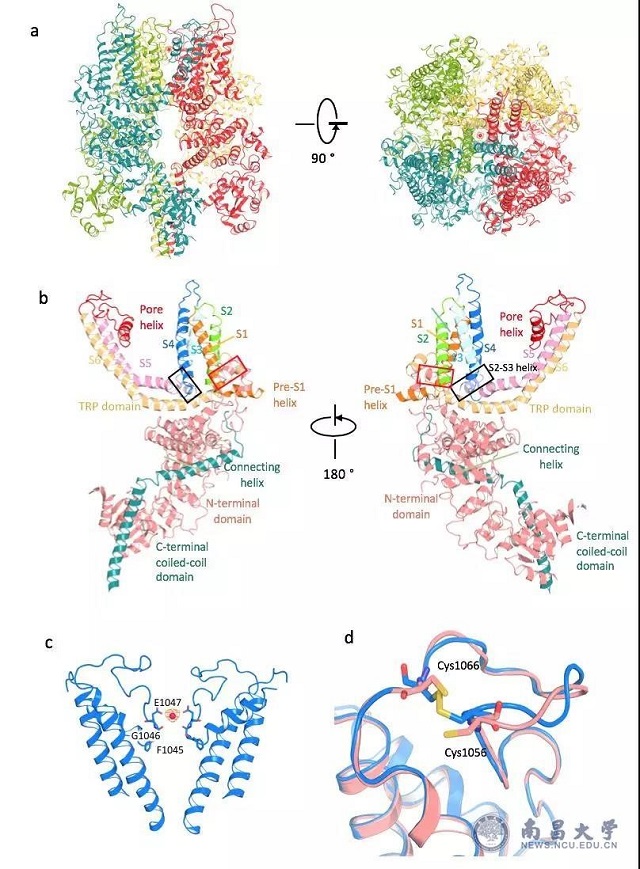
This study resolves the high-resolution structure of magnesium ion channel proteins in mammals and reveals the binding sites of magnesium ions at the center of the TRPM7 selective filter for the first time. Unlike sodium and potassium ions, which have a clear two hydration layers, so it is generally believed that the complete dehydration of magnesium ions through TRPM7 requires very large amounts of energy. The study found that the magnesium ion in the TRPM7-Mg2+ structure is only partially hydrated. The carbonyl group on the Gly1046 carbon skeleton of the selective filter and the carboxyl group of Glu1047 can replace the water of the second hydration layer and compensate the energy for the dehydration of the hydrated Mg2+ into the channel. Secondly, the lower gate of the channel has two constrictions, Ile1093 and Asn1097, respectively, wherein the side chain of Asn1097 from each monomer forms a polar ring at the entrance of the hole to regulate Mg2+. It is worth noting that the unique disulfide bond structure of the extracellular loop of TRPM7 is required for its function and may be related to the activation mechanism unique to the TRPM family. This study reveals the molecular mechanism of magnesium ion regulation in mammals and lays the foundation for understanding the structure of the TRPM subfamily, which will greatly promote and accelerate the development of new anti-stroke drugs.
 Personal profile: Zhang Jin, postdoctoral fellow at Harvard University, is currently a distinguished professor and doctoral supervisor at the Basic Medical School and Biomedical Research Institute of Nanchang University. Professor Zhang Jin has been engaged in the study of the structure and function of drug targets related to human major diseases for a long time. He has studied the analysis of major disease-related membranes by multi-disciplinary methods such as medicinal chemistry, pharmacology and electrophysiology through X-ray crystallography and single-molecule cryo-electron microscopy. The three-dimensional structure of proteins (including G-protein coupled receptors and ion channels), and the development of novel targeted drugs based on structure. In recent years, he has published papers in Nature (2), Nature Communications, PNAS (2), and Proteins (2) as the first author or correspondent author and in international journals such as Nature Communications, elife, and Developmental Cell as co-authors.
Personal profile: Zhang Jin, postdoctoral fellow at Harvard University, is currently a distinguished professor and doctoral supervisor at the Basic Medical School and Biomedical Research Institute of Nanchang University. Professor Zhang Jin has been engaged in the study of the structure and function of drug targets related to human major diseases for a long time. He has studied the analysis of major disease-related membranes by multi-disciplinary methods such as medicinal chemistry, pharmacology and electrophysiology through X-ray crystallography and single-molecule cryo-electron microscopy. The three-dimensional structure of proteins (including G-protein coupled receptors and ion channels), and the development of novel targeted drugs based on structure. In recent years, he has published papers in Nature (2), Nature Communications, PNAS (2), and Proteins (2) as the first author or correspondent author and in international journals such as Nature Communications, elife, and Developmental Cell as co-authors.




