It was reported that (by the School of Chemistry and Chemical Engineering) recently, the research team led by Professor Chen Yiwang and Professor Tan Licheng from the School of Chemistry and Chemical Engineering of NCU has achieved a new breakthrough in the field of electrochemical energy storage. They innovatively proposed the novel concept of “Water-shielding electric double layer and stable interphase engineering”, effectively addressing the critical scientific challenge of interface failure in aqueous zinc-ion batteries. This research finding, entitled “Water-shielding electric double layer and stable interphase engineering for durable aqueous zinc-ion batteries”, has been published in the prestigious international journal Nature Communications. The first author of the paper is Peng Zhongyou, Assistant Research Fellow from the School of Chemistry and Chemical Engineering, while Professor Chen Yiwang and Professor Tan Licheng serve as the corresponding authors. NCU is the primary affiliation and corresponding institution.

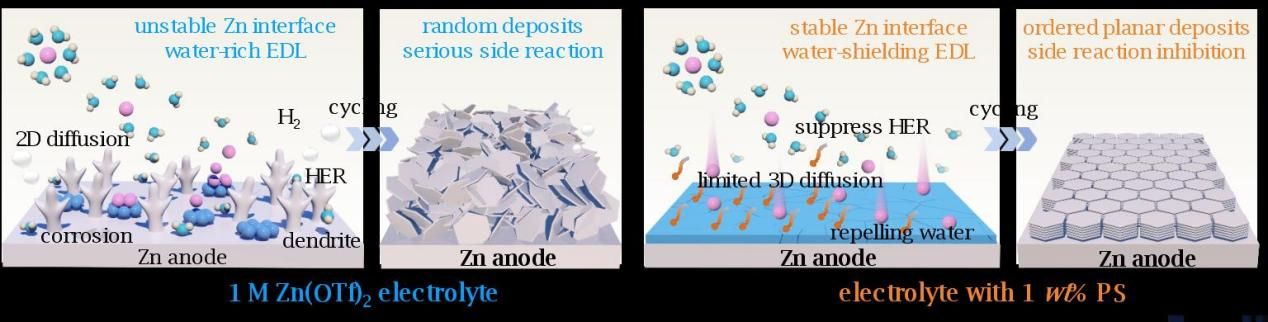
Aqueous zinc-ion batteries are highly competitive candidates for large-scale energy storage applications due to the advantages of the zinc metal electrode, including its high theoretical capacity, low electrochemical potential, natural abundance, and low cost. However, their commercialization is hindered by interfacial issues: uncontrolled zinc dendrite growth, chemical corrosion, the hydrogen evolution reaction (HER), and low zinc utilization, leading to Coulombic efficiency and cycle life that fall short of industrial benchmarks. To tackle this key scientific problem of interface failure, this study pioneers the innovative concept of “Water-shielding electric double layer and stable interphase engineering”, and also proposes a strategy utilizing trace amounts of polysorbate electrolyte additive, systematically revealing the microscopic mechanism by which polysorbate molecules form an oriented interfacial layer through chemical adsorption, while simultaneously inducing the formation of an organic-inorganic hybrid solid electrolyte interphase (SEI). This synergistic regulation strategy enables ultra-long stable cycling of the zinc electrode for 450 hours at an industrial-level current density of 40 mA cm⁻⟡, breaking through the technical bottleneck in this field. It provides an original solution to the commercialization challenges of rampant zinc dendrite growth and parasitic HER, setting a new record in the field. The study also proposes an interfacial water activity factor model, clarifying the structure-activity relationship between the hydrogen bond network of water molecules in the electric double layer and the kinetics of side reactions. This offers new insights for the development of high-safety, low-cost energy storage technologies.
This work has received substantial support from the Key Special Projects of the National Key Research and Development Program of China, the National Natural Science Foundation of China (NSFC), the Postdoctoral Fellowship Program of CPSF, and the China Postdoctoral Science Foundation (CPSF).
Paper link:
https://www.nature.com/articles/s41467-025-59830-y




